The tightening up of new French and international normative and regulatory requirements has increased the challenges involved in designing and manufacturing cosmetic products. As a long-term partner of the cosmetics industry, Consultys deploys an offer to suit the needs of the sector’s industrial operators.
The European regulatory environment, the GMPs, the risk management and the new ISO microbiology reference systems are all areas in which cosmetics companies must be particularly vigilant.
So that you can devote all your energy to your core business, cosmetic innovation, Consultys proposes a range of expert services for this sector. From formulation to the first safety and quality tests, tests for product release and the marketing of cosmetic products, our specialist consultants support projects and help to create a favourable industrial environment for all stages of development.
- Research & development
- Processes
- Design engineering
- Commissioning-Qualification-Validation
- Quality
- Production

Research & development is a highly strategic area for all industrial players in the Life Sciences sector.
Research & development officers assist the pharmaceutical development of products, from pre-clinical and clinical stages to the final stage of submission of the regulatory dossier, in compliance with applicable regulations and Good Laboratory Practice.
Position examples: product development officer / manager, research officer, formulation engineer, R&D assistant, R&D project officer, mechanical design project manager, etc.

Pharmaceutical and biotech processes concern all the operations leading to the transformation of raw materials into finished products and are therefore essential to the final drug production.
The Process Project Manager is responsible for the design, implementation, monitoring, evaluation and optimisation of processes for the Research & Development and Production departments.
He/she performs process studies in compliance with health and safety norms and applicable regulations.
Position examples: process study supervisor, process engineer, process development supervisor, process validation supervisor, cleaning validation supervisor, bioprocess engineer, process equipment criticality supervisor, industrial transposition engineer, process transfer project manager, bioprocess experts, etc.

Design engineering demands technical experts trained in specific regulatory matters.
The engineering project manager is responsible for the feasibility study phase and the project construction phases.
He/she deals with the overall project management, i.e. budget monitoring, overall schedule management, contract management and reporting throughout the project.
Position examples: equipment project supervisor, utilities/HVAC project supervisor, infrastructure and equipment project manager, engineering/automation project manager, packaging design engineer, packaging supervisor, new build engineer, revamping project supervisor, building project supervisor, etc.

These stages verify that the equipment or premises fulfil quality and functional constraints in terms of operation and efficiency during their design, construction, commissioning and use.
The commissioning supervisor coordinates and commissions the equipment, manages negotiations with suppliers, conducts risk and criticality analyses.
The qualification / validation officer drafts and coordinates the strategy to meet the organisational requirements of industrial operations, defining the master plan for validation, protocols and reports in strict compliance with health and safety conditions and applicable regulations.
He/she may work on laboratory equipment or production/packaging equipment or units, utilities, computer systems, etc.
Position examples: commissioning supervisor, qualification supervisor, validation supervisor, validation plan manager, computer system validation supervisor, environmental qualification supervisor, transport qualification supervisor, pharmaceutical process and equipment engineer.

In the past few years, quality controls have become increasingly strict and industrial operators are now facing major quality challenges. In the coming years, policies will be affected by the computerisation and digitalisation of expertise.
The quality assurance officer ensures the quality, efficacy and safety of patients throughout the various stages of the healthcare product life cycle in compliance with very strict regulations. Being responsible for deploying the strategy to achieve the objectives, he/she drafts the action plans, ensures correct implementation of the quality procedures with respect to applicable regulations and ensures monitoring of the quality indicators implemented and all corrective and preventive actions.
He/she defines the quality policy within the teams, providing training for the personnel concerned.
Position examples: system quality assurance supervisor, operational quality assurance supervisor, anomaly investigator, CAPA and quality deviation supervisor, supplier quality assurance supervisor, risk management coordinator, sterility assurance supervisor, data integrity officer, SISA compliance officer, analytics methods development supervisor, stability coordinator, quality project manager, etc.

Production areas are starting to seek new technical skills in automated systems, IT and mechanics to respond to the challenges of computerising industrial equipment.
The production coordinator implements the production strategy according to pre-defined objectives, in compliance with regulations and quality requirements, health and safety rules and within pre-determined deadlines and cost limits.
He/she ensures smooth operation of the production chain: manufacturing, quality, scheduling, methods, procurement.
He/she supervises and leads the production teams, coordinating actions in collaboration with the other departments and reporting to Senior Management.
Position examples: production planner, manufacturing manager, production manager, etc.
Supply chain activities optimise storage costs while securing delivery lead times for industrial operations. Specific planning and flow management skills based on integrated management software solutions and project management expertise are essential.
The Supply Chain coordinator helps to implement raw material and product flow management policies, guaranteeing the cost-lead time ratio and product quality.
He/she determines the priorities for dossiers and products, bearing in mind regulatory and industrial constraints and ensuring scheduling and procurement requirement anticipation to optimise deliveries.
He/she creates performance indicators and monitors them regularly, promoting different themes within the team.
Position examples: supply chain engineer, logistics project supervisor, logistics quality supervisor, procurement manager, planner, etc.

Preserving the operability of equipements and production tools is fundamental: maintenance is therefore a key area in which preventive action is gradually taking over from predictive methodologies. Statistical analyses enable measurement of failure or breakdown occurrence according to equipment use.
The maintenance coordinator is responsible for maintenance, performance improvement, equipment upgrading and safety.
He/she guarantees the availability and compliance of work equipment and facilities in collaboration with the quality, manufacturing and supply chain departments, and provides reports based on the indicators monitored.
He/she heads work groups, deploys action plans with the various technical parties involved and is a member of the steering committee.
Position examples: maintenance officer, maintenance reliability engineer, technical services supervisor, etc.
Biostatisticians have the scientific and technical skills to analyse and interpret large volumes of data. Recent technological progress enables increasingly detailed and complex simulations and models; new analysis tools now offer a wide range of analysis possibilities for industrial players.
The biostatistician participates in pre-clinical, clinical and epidemiological studies, the design and development of biostatistics methodologies, the implementation of biological studies, the analysis, interpretation and publication of results, and ensures statistical monitoring.
Position examples: statistician, biostatistician, statistical study supervisor, data analyst, CMC statistician, etc.

In relation to the group’s overall strategy and requirements, the regulatory affairs department guarantees the development, registration and exploitation of products in compliance with all the local and international regulations applicable to the territories in which the products are distributed.
The regulatory affairs officer performs all the actions involved in obtaining and maintaining the Marketing authorisation and CE marking, while guaranteeing the application of regulations throughout all stages of the product life cycle.
Position examples: regulatory affairs officer, technical regulatory affairs officer, regulatory affairs coordinator, CMC compliance project manager, labelling pharmacist, etc.
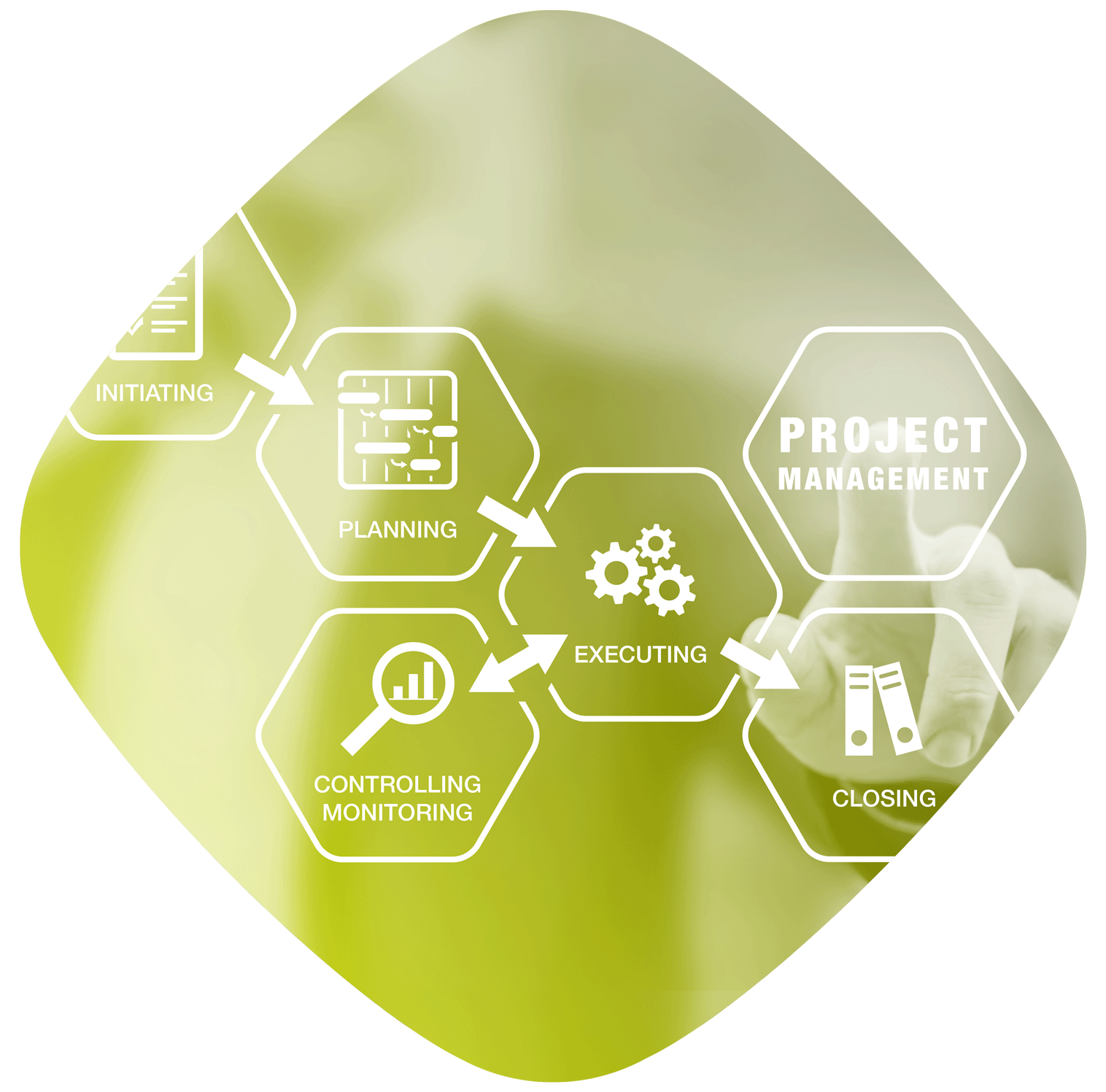
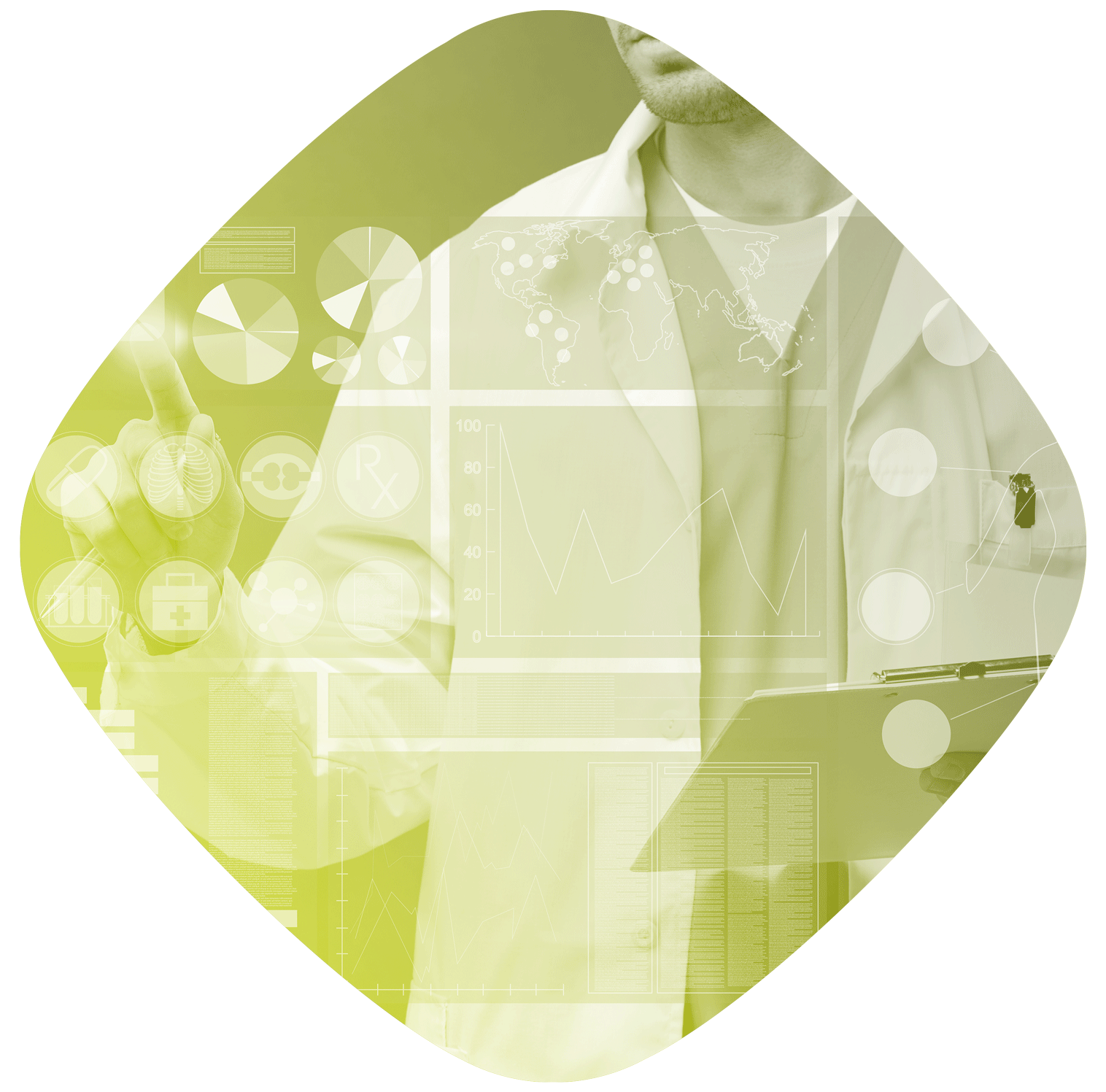
Project management is an essential skill in a business: it enables optimisation of the costs and resources allocated to each project developed.
The Project Manager Office defines and develops project management strategies and procedures to implement the associated methodologies from a global perspective; it also reports to the various parties involved.
It ensures operational coordination, manages project performance monitoring and guarantees the coherency and alignment of the portfolio with the objectives defined.
Position examples: project planner, project organisation manager, project officer / coordinator / management officer, analytics programme coordinator, site quality coordinator, etc.

Continuous improvement concerns all activities and industrial sites.
The Lean Management engineer ensures and develops sustainable methods by deploying the Lean Management standards to implement and develop continuous improvement methods for processes in order to optimise production.
He/she defines and monitors the performance indicators and implements actions to improve the efficiency of processes and equipment; he/she reports on all these activities.
Position examples: continuous improvement engineer, Lean engineer, process and continuous improvement engineer, Lean project manager, continuous improvement manager, etc.
Consultys
is recruiting
Are you an engineer, pharmacist or researcher?
Do you have significant experience in the pharma / biotech / MD / Cosmetics industrial sectors?
Do you share our values?
Would you like to work on strategic industrial projects?
Why not join us and make the most of the following opportunities!